Molarity of Pure Water – Measures of the concentration of a chemical solution include molarity and molality. The main distinction between the two is volume and mass.
While molarity is concerned with the moles of a solute with respect to the volume of a solution, molality discusses the moles of a solute in relation to the mass of a solvent.
Continue reading to find out more about molarity and molality, including a comparison of the two concepts, their meanings, and equations.
Molality definition
The quantity of a material dissolved in a given mass of solvent is known as its molality (m), also known as molal concentration. The moles of a solute per kilogramme of a solvent is how it is defined. [Revised on May 4, 2020
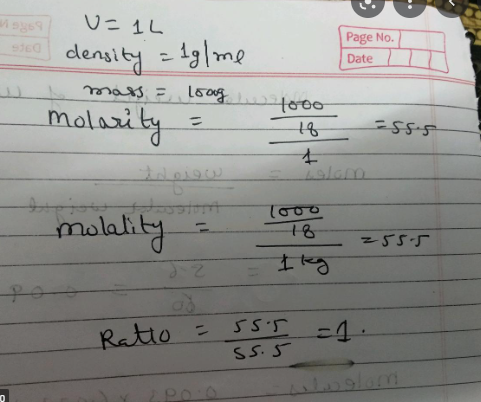
Formula and units for molality
Molality is measured in m or mol/kg.
Molality formula
m = kilogrammes of solvent/moles of solute
Definition of molarity
The amount of a substance in a specific volume of solution is known as its molarity (M). The number of moles of a solute per litre of a solution is known as molarity. The molar concentration of a solution is another name for molarity.
Formula and units for molarity
Molarity is measured in units of M or mol/L. One molar is used to describe a 1 M solution.
Molarity formula
M = litres of solution/moles of solute
Molality versus molarity
The distinction between a solution and a solvent is a crucial one between molality and molarity.
The moles of a solute divided by the sum of the litres of the solution is known as the molarity. Both the solute and the solvent are present in the solution.
Molality, on the other hand, is the proportion of a solute’s moles to a solvent’s kilogrammes. Keep in mind that the mass used as the denominator only includes the solvent and not the solute.
-The amount of the solute in moles per litre of solution is known as molarity.
Molarity= liquid volume in litres
Number of solute moles
We are informed that water has a density of 1g/ml. This is equivalent to $1 Kg/L.
Therefore, 1L of water will have the following mass:
= Water density Volume=1LKg×1L=1Kg=1000g
So, the number of moles in 1 kilogramme of water is equal to molar mass mass=181000=55.56
As a result, the molarity of water is =1L
55.56moles=55.56 M

To get the molarity of pure water, we must convert the unit of water density from gm/ml to Kg/L. This is necessary to ensure that the units are the same since the molarity is computed per litre of the solution.
Video of Cliff Jumping and Rafting in Rishikesh
To Book Rafting Camping Tour in Rishikesh and Jim corbett Park Or Rajaji National Park Stay and Jeep Safari
Direct WhatsApp Contact – CLICK HERE
Read our other Articles – Best River Side Resort in Rishikesh
Subscribe to Our YouTube Channels for more Rajaji National Park Information